The Basic Oxygen Steel Making Process
The Basic Oxygen Steelmaking (BOS) process is the steel refining process commonly used today where the molten iron from the blast furnace is mixed with scrap steel to aid the cooling process as very high temperatures are produced in BOS process.
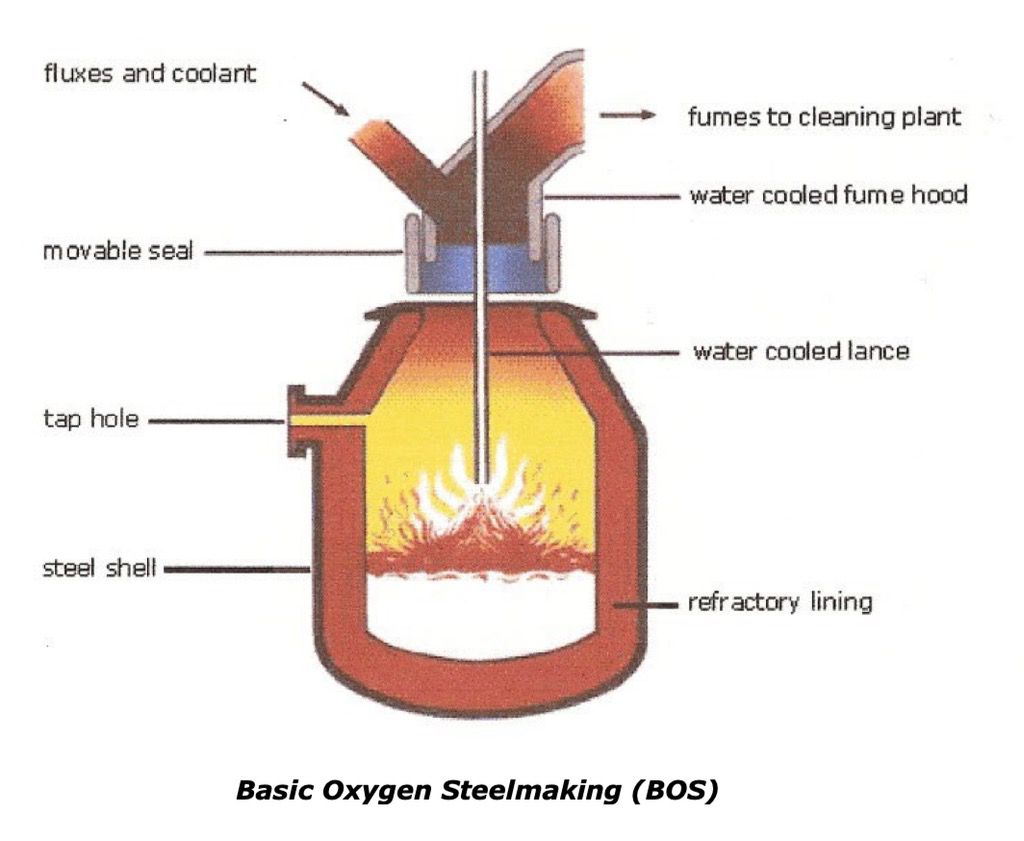
Most of the impurities which are contained in the pig iron are removed by the oxidation process, where oxygen is blown through the molten metal by a water cooled lance and reacts with the impurities as it passes through. The carbon content is lowered and changes the pig iron to a low carbon steel. However the oxygen also reacts with the iron to produce dissolved iron oxide which would release gases on cooling and cause unsound castings or ingots. The addition of manganese transfers the oxygen to a manganese oxide which is removed with the slag.
The steel is tapped from the furnace when it is at the correct temperature and composition. The furnace is tilted and the molten metal is run out via the tap hole into a ladle. Once the steel has been removed, the furnace is turned upside down for the slag to run into another ladle which is further used in the production of cement or in building roads. Quantities of 250 tons of steel may be processed in a little as 40 minutes.
The process generates large quantities of heat so up to one fifth of the charge is made up of steel scrap partly to control temperature.
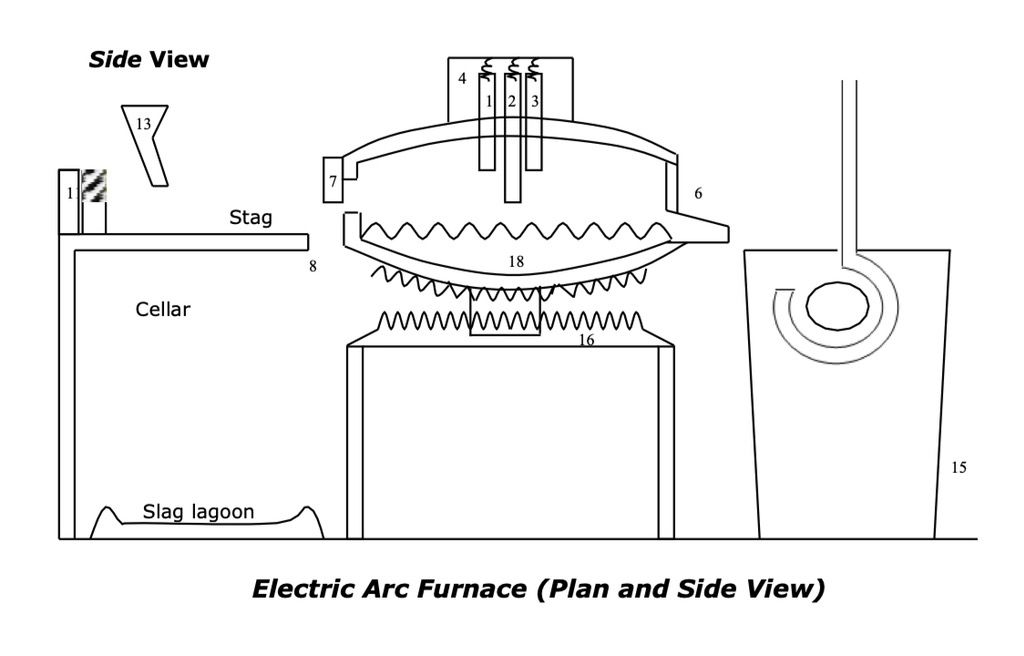
Electric arc furnaces are used for smaller quantities of high grade steels. The electric arc furnace usually melts scrap metal that has been shredded which has a known content and has already been refined as above and so can control final analysis more closely.
The furnace is charged with the scrap metal, the roof of the furnace is then swung back over the furnace to allow meltdown to start. The electrodes are lowered onto the scrap metal and an arc is struck which then starts the melting process. Once the temperature and chemistry of the steel is correct it is tapped off into ladles through tilting the furnace.
Further reduction in gases can be made by subjecting the liquid to a vacuum to draw off the gases. This is called vacuum degassing.
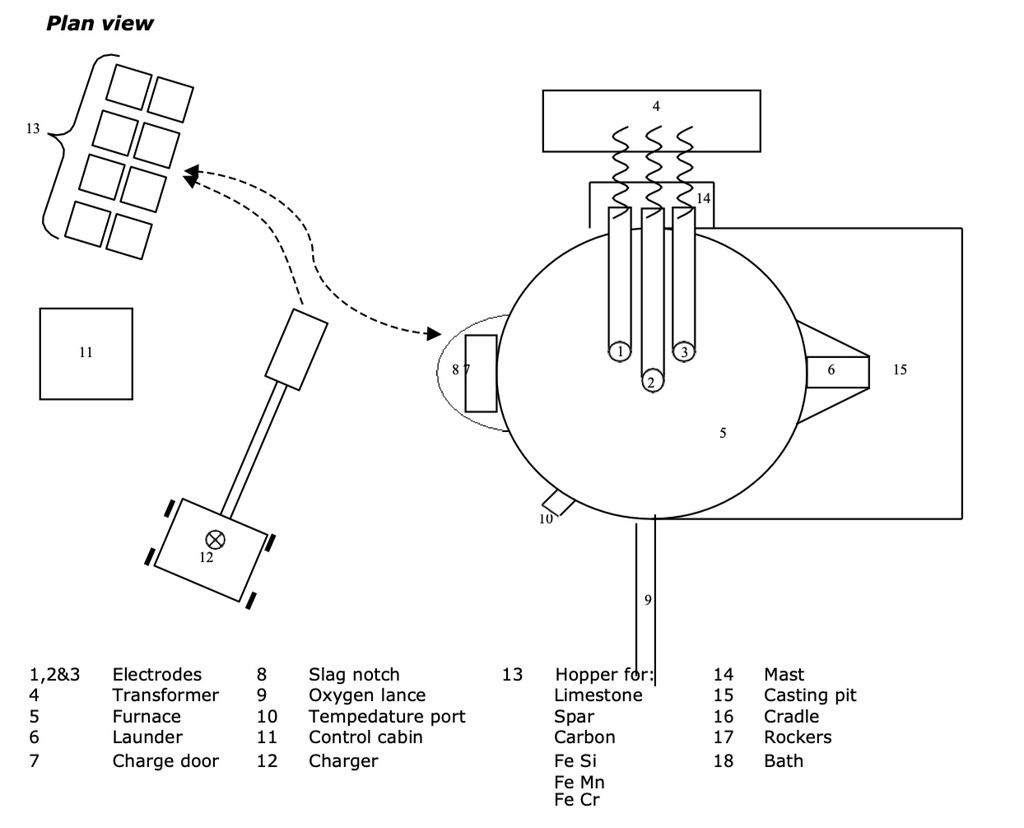
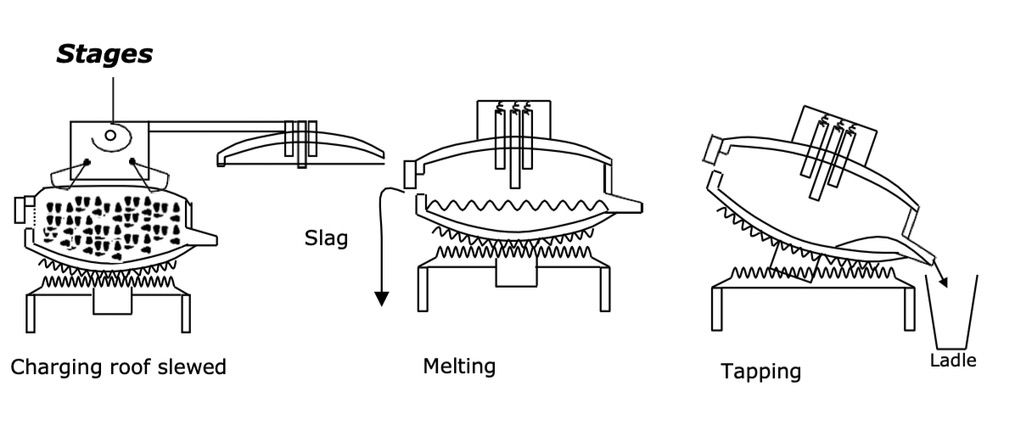
Charging
Usually 2 baskets – first part melted, then second charged.
Melting
Melting is accomplished using oxygen and fuel + arc to melt the charge. A carbon boil is used to achieve 0.5-0.6% carbon content. During the melting process Si is reduced to SiO2, Mn to MnO and P is reduced to P2O5. These will be contained in the slag. The boil purges the melt of N₂ and H₂.
After the oxidising stage the slag is removed and the bath ‘blocked’ (deoxidised) with Si or Mn or Al or a combination of these. Desulphurisation can then be achieved in the ladle with CaO resulting in CaS.
Note: electric arc furnaces are very versatile with range of steels from low C to stainless steels and super alloys.
Si = Silica
O = Oxygen
Mn – Manganese
P = Phosphor
N – Nitrogen
H = Hydrogen
Al = Aluminium
Ca = Calcium